Chinese Team Discovers New Mechanism of Ventricular Remodeling After Myocardial Infarction, Showcased in Circulation Journal
Release Time:
2024-03-05 15:26
Recently, a research paper published in "Circulation" by a team led by Zhang Ye and He Shufang from the Department of Anesthesiology and Perioperative Medicine at the Second Affiliated Hospital of Anhui Medical University and Hu Ji from the ShanghaiTech University revealed a new mechanism of post-infarction ventricular remodeling mediated by the mechanosensitive channel Piezo1.
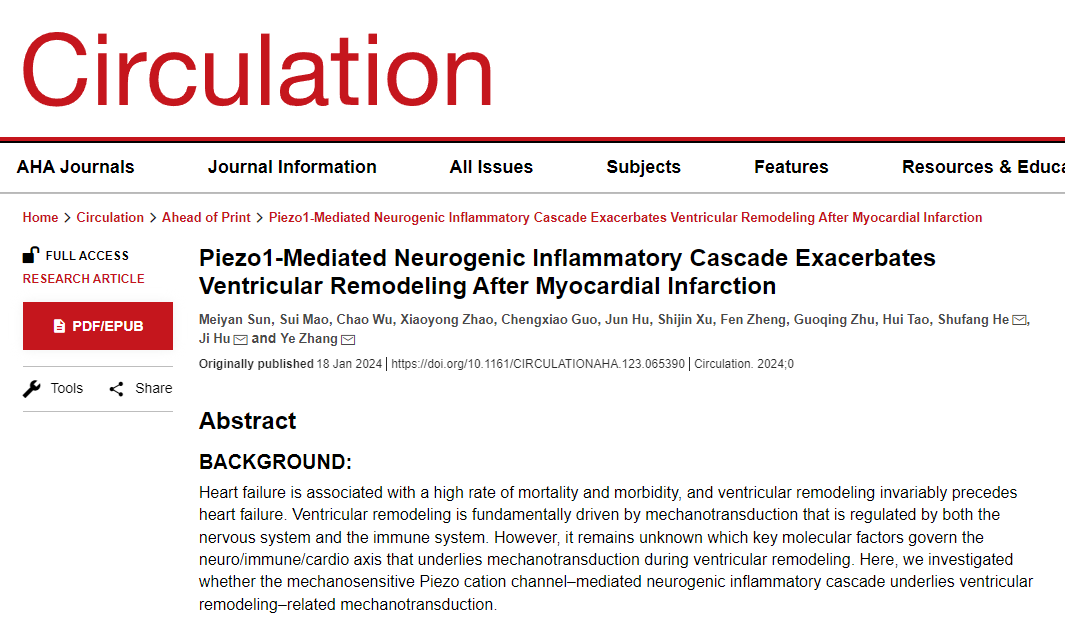
《Circulation》 (Impact Factor = 37.8) is a top international journal in the field of cardiovascular disease research. The team's significant findings provide new targets for preventing and treating post-infarction ventricular remodeling or heart failure, creating profound theoretical and clinical value with far-reaching implications.

Heart failure (HF) is a severe and terminal stage of cardiovascular disease, with high disability and mortality rates. With the development of aging population, the incidence of heart failure is gradually increasing, becoming an increasingly important global public health issue. Myocardial infarction (MI) is one of the most common and important causes of heart failure worldwide. The occurrence of heart failure after myocardial infarction significantly increases the risk of death for patients, making early prevention and treatment strategies crucial.
The occurrence of heart failure after myocardial infarction is not without trace. In the mid-1990s, it was recognized that ventricular remodeling is the basis for the development and progression of heart failure. The pathological process involves abnormal mechanical signal transmission and is subject to dual regulation by the nervous and immune systems. However, the key molecular factors controlling the neuroimmune axis as the basis of mechanical transmission remain obscure.
Venture into the Unknown
In this study, the research team focused on the Piezo1 channel in the thoracic dorsal root ganglion (TDRG) where the cell bodies of cardiac sensory neurons are located. Piezo1 is a specific mechanosensitive ion channel, and its discovery in 2021 was awarded the Nobel Prize in Physiology or Medicine. The study elucidated a novel neuroimmune mechanism of post-infarction ventricular remodeling mediated by Piezo1/IL-6, a mechanism involving neurogenic inflammation.
The researchers found that the expression level of Piezo1 in TDRG significantly increased during the process of post-infarction ventricular remodeling and was strongly correlated with disease severity. They further discovered that the activation of Piezo1 in TDRG can induce calcium influx in neurons, leading to increased release of the inflammatory factor IL-6. These neurogenic IL-6 molecules reach the local myocardial tissue through axoplasmic transport and, by activating the cardiac IL-6 receptor and the STAT3 inflammatory signaling pathway, promote post-infarction ventricular remodeling. By using RNA interference, neuron-specific knockout, and intrathecal administration of neutralizing antibodies to block the Piezo1/IL-6 signaling cascade in TDRG, the researchers were able to inhibit cardiac IL-6/STAT3 inflammatory signaling, thereby improving post-infarction ventricular remodeling.
The research process was based on the Department of Anesthesiology of the Second Affiliated Hospital of Anhui Medical University.
The image was extracted from the original paper.
See more possibilities
During the study, the team established a chronic myocardial infarction model in rats by ligating the left coronary artery. They used slow virus-mediated TDRG-specific Piezo1 gene knockout in rats and adeno-associated virus-PHP.S-mediated TDRG neuron-specific Piezo1 gene knockout in mice to investigate whether Piezo1 in TDRG plays a functional role in ventricular remodeling.

Throughout the experiments, the research team used the professional scientific ultrasound system VINNO 6Lab to perform detailed and comprehensive observations and monitoring of changes in cardiac function in rats and mice. This included measuring left ventricular function, such as ejection fraction, left ventricular end-diastolic diameter, left ventricular end-systolic diameter, and other parameters, providing reliable imaging data support for the study.
As a member of the VINNO Lab Ultrasound Series designed specifically for rodents, VINNO 6Lab possesses hardware and software specifically tailored for mice and rats and offers outstanding cardiac application solutions. It can assist researchers in completing precise and diverse research tasks, including cardiovascular research and gene expression pattern analysis, tumor research, and drug development.

The specialized cardiac model for rats and mice

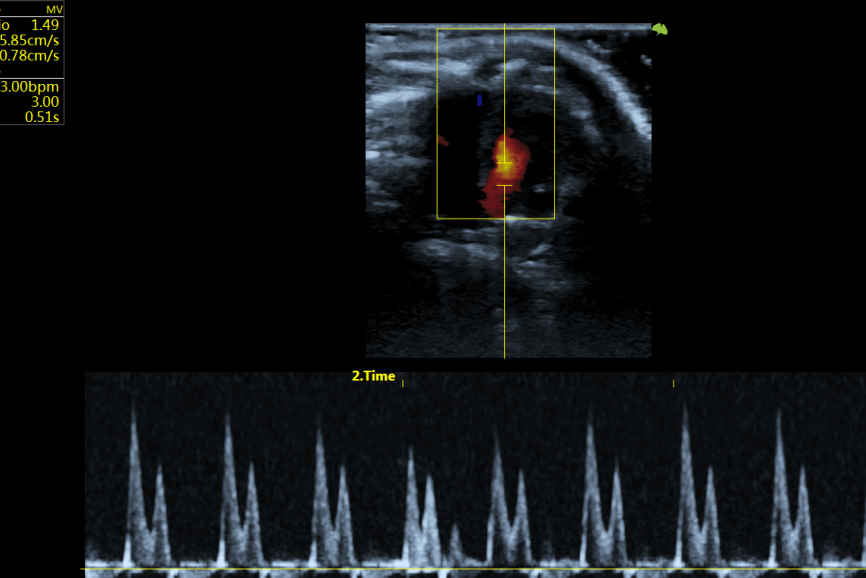
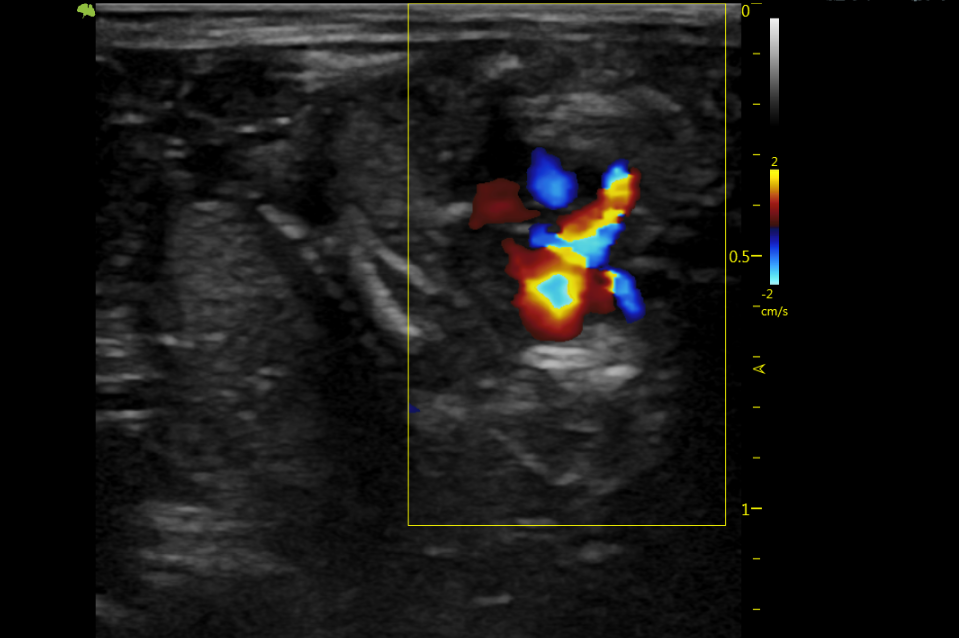
Display of cardiac application images





